
Compliance

California authorities say predominance of retailers licensed to sell hemp are compliant with ban published effective on 3 October through emergency regulation. But hemp industry’s contesting the regulation in state court, arguing state authorities inappropriately used emergency action to change state law on hemp products legislature passed in 2021.

The Federal Trade Commission’s Consumer Protection head Sam Levine says bipartisan leaders at the state and federal levels are taking on consumer protection issues like never before and using the FTC’s approach as a model for legislation.

Reorganization creating in Human Foods Program makes the work of additional staff available for dietary supplement office programs. FDA’s “got a little tiny workforce” for “a huge industry,” says Commissioner Robert Califf.

HiSmile, which is appealing a National Advertising Division case decision arguing that online advertising’s global footprint demands evaluation of ad claims by international standards, has been referred to the FTC for declining to participate or comply in recent NAD cases brought forward by Oral Essentials and Procter & Gamble.

Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.

Attorneys discuss potential impacts on consumer health products industry from Supreme Court’s “Loper Bright” decision in June on litigation brought by two fisheries, Loper Bright v. Raimondo and Relentless v. Department of Commerce.

Committee’s report published with FY2025 appropriation states a different approach to establishing FDA regulation of non-drug products containing hemp as a derivative of cannabis de-scheduled as controlled substance in the US since 2018.

The US FDA has issued its final guidance defining manufacturer behaviors it deems as hampering the agency’s ability to conduct an inspection. In a previous draft guidance, the agency expanded its longstanding policy on inspections of drug companies to include device makers as well.

Finished product following Reagan-Udall food safety programs review establishes Human Foods Program in commissioner’s office while also realigning centers, offices and divisions across agency to improve collaboration with regulatory affairs, which conducts facility inspections and other field operations.

Without additional funding, FDA’s ORA faces challenges in retaining and hiring staff, which will impact inspections, says office chief Michael Rogers.

EAS Consulting Group’s John Bailey continues to chronicle regulatory developments under the Modernization of Cosmetic Regulations Act. In this installment, he outlines industry stakeholders’ questions and concerns related to 2023 announcements from the US FDA and provisions of the law that became effective (if not yet enforced) at year-end.

FTC Division of Marketing Practices assistant director concerned direct selling self-regulation group’s guidance “will encourage deceptive conduct and facilitate deceptive earnings claims.” DSA president says the concerns might reflect the thinking of agency’s staff more than the intent of the agency’s regulations.

Some advertisers try to avoid scrutiny of regulators and the National Advertising Division when making health-related claims by making a claim “softer.” Annie Ugurlayan, NAD’s assistant director, offers advice on assuring substantiation and a health claim match.
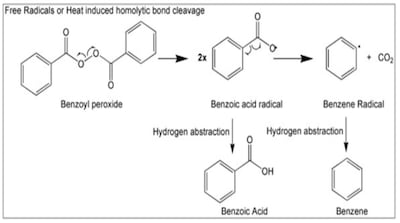
Valisure recommends FDA request recall and halt sales of products containing benzoyl peroxide due to its propensity to form the benzene. Products include numerous OTC monograph topical formulations as well Rx drugs with an acne indication available through approved applications.

Nearly 1,300 rodents exterminated at Arkansas warehouse after FDA investigation found lack of climate control in store of food and OTC drugs requiring dry, room-temperature conditions. Firm pleads guilty in largest monetary criminal penalty in US food safety case.

AI has a place in the FTC's proposed rule to ban fake consumer reviews and testimonials in online advertising in the US for a reason, one that BBB National Programs also acknowledges.

BBB National Programs found FTC’s proposed Trade Regulation Rule on the Use of Consumer Reviews and Testimonials largely effective with some certainty on whether product reviews or customer testimonials are violations of its false advertising standards. But it =expects some clarifications may come in final rule.

Speakers scheduled for hearing are Fake Review Watch, which says it’s “dedicated to exposing the massive corruption of online consumer review platforms”; University of California-Davis researchers who found widespread fraud; and Interactive Advertising Bureau, which says its members account for 86% of online advertising expenditures in the US.

Former DOJ official notes this can be a difficult question before government initiates an investigation unless there is an obvious problem. DOJ official describes benefits of cooperation and how data analytics have changed the way the department identifies and vets cases.

Agency proposal, now under HHS review, further reduces chances of losing another whistleblower complaint by taking ORA out of the loop, while proposing a new name for its slimmed-down field organization: Office of Inspections and Investigations. Meanwhile, an agency veteran, Michael Rogers, will lead the revamped organization as associate commissioner.