
Regulation

Perrigo is among the supporters of a campaign to get a clause of Germany's Medicines Advertising Act removed, which bans the advertising of the OTC morning after pill.
Supreme Court’s Loper Bright “decision is central to this case,” says Jonathan Emord, attorney representing Alliance for Natural Health USA and Meditrend Inc. in a complaint filed in US District Court for the District of Columbia.
FDA’s OTC office director details a 2023 guidance as opening doors for NRT innovation at recent public meeting, but researchers and an industry executive note the most recent approval in the US for an innovative NRT was more than 20 years ago and say FDA isn’t allowing sufficient flexibility for approvals of new products or indications other than cessation related to quitting smoking.

FDA tobacco programs chief emphasizes moving smokers to lower risk alternatives and NIDA executive encourages proposals for e-cigarettes as nicotine replacement treatments. FDA also seizes $76m in unauthorized e-cigarettes.
Slow adoption of alternatives to animal testing in the current decentralized regulatory framework.

Requests for “enforcement actions are not within the scope of FDA’s citizen petition procedures,” CDER says, rejecting petition dosing device firm Parenteral Technologies submitted as it prepares for workshop on Pediatric Research Equity Act requirements for OTC NDA sponsors.
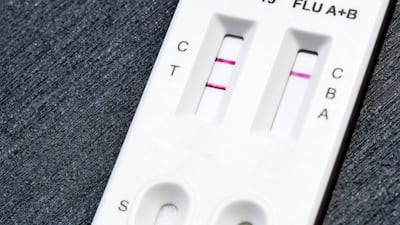
FDA grants de novo authorization to Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first OTC flu test to be cleared outside the emergency use pathway.

UK pharma also reaches agreement in principle, subject to DoJ approval, to pay $70m to resolve a whistleblower complaint filed by Valisure, the testing lab which in 2019 raised concerns about a potential link between the use of drugs containing ranitidine, a histamine-2 blocker, and cancer.

NDAs for additional OTC products containing acetaminophen and/or NSAIDs and indicated for use by children between 2 and less than 12 years old would trigger compliance by application sponsors with the act passed in 2003 to address lack of pediatric use information in drug labeling.

Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.

Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”

Nonbinding forecast, fourth for the program, includes risks associated with codeine-containing cough medicine as a topic FDA will include in ongoing evaluation of GRASE for pediatric cough cold drug products marketed under monograph for cold, cough, allergy, bronchodilator and anti-asthmatic OTC drugs.
Council for Responsible Nutrition contends New Jersey bill, which Assembly Health Committee amended with substantial language to make restrictions more stringent on 23 September, is targeted as inaccurately as restrictions in New York effective in April.
October will see the bricks-and-mortar launch of the first FDA-approved OTC treatment for erectile dysfunction in the US by Haleon. HBW Insight catches up with the company's North America president Lisa Paley to find out what Haleon has planned for Eroxon's arrival in stores.

Following Energy and Commerce Health Subcommittee hearing about FDA’s human food and tobacco programs on 10 September, gap between what the trade groups, committee leadership and the FDA each want more of doesn’t appear to be shrinking.

This Thursday (26 October) the United Nations will discuss whether OTC antimicrobial products should be restricted to prescription-only status by UN member states. In advance of this, we catch up with UK association CEO Michelle Riddalls, to learn about the PAGB’s role in crafting the global consumer healthcare industry’s response.

FDA approves Flumist consumers can use at home for prevention of flu caused by virus subtypes A and B in individuals 2 through 49 years old in a proposal submitted by AstraZeneca’s MedImmune.

In addition to commentary and information about the OTC drug, dietary supplement and cosmetic industries, speakers offered candid remarks on subjects well known across the consumer health industry or well recognized by consumers.

Filed on 20 August by Pensacola, FL-based law firm Aylstock, Witkin, Kreis & Overholtz (AWKO) on behalf of an unnamed client, the petition points to benzene study data unconnected to Valisure on unheated benzoyl peroxide products, but is light on details of how it was conducted.

Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.