
Compliance

Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.

Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.

Reorganization creating in Human Foods Program makes the work of additional staff available for dietary supplement office programs. FDA’s “got a little tiny workforce” for “a huge industry,” says Commissioner Robert Califf.

Report from European Commission's Alert and Cooperation Network finds EU consumers are being deceived by companies marketing supplements making unauthorized health claims and containing unapproved ingredients.

HiSmile, which is appealing a National Advertising Division case decision arguing that online advertising’s global footprint demands evaluation of ad claims by international standards, has been referred to the FTC for declining to participate or comply in recent NAD cases brought forward by Oral Essentials and Procter & Gamble.

Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.

Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.

Attorneys discuss potential impacts on consumer health products industry from Supreme Court’s “Loper Bright” decision in June on litigation brought by two fisheries, Loper Bright v. Raimondo and Relentless v. Department of Commerce.

Analysts suggest more and larger investments than president’s recent $1m and $5m private placements needed to last long enough to establish sales of its functional beverage sufficient to turn a profit. Results expected soon from clinical trials on “detoxifying the alcohol out of” users’ bodies.

CRN gained approval of motion for expedited briefing when it notified Second Circuit it would appeal District Court for Southern New York’s ruling against its motion for preliminary injunction against state law prohibiting sales to minors of supplements and OTC drugs containing ingredients labeled or promoted for weight loss and bodybuilding.

The US FDA has issued a final rule allowing the agency to destroy some medical devices that have been refused entry into the US. The rule takes effect 1 July.

Finished product following Reagan-Udall food safety programs review establishes Human Foods Program in commissioner’s office while also realigning centers, offices and divisions across agency to improve collaboration with regulatory affairs, which conducts facility inspections and other field operations.

A generic of Reckitt’s Nurofen Rapid can no longer be sold in the EU following a decision by the European Commission.
Court ruling that state’s restrictions “may very well regulate protected speech”’ allows CRN “to move forward on the merits of the case,” says CEO Steve Mister. CRN’s allegations “plausibly support the inference that the [restriction] might very well regulate protected speech,” judge writes.

Without additional funding, FDA’s ORA faces challenges in retaining and hiring staff, which will impact inspections, says office chief Michael Rogers.

Southern New York district judge rejects CRN’s arguments to block regulation from taking effect, but agrees with trade group and its members that they have standing to challenge the regulation. CRN complaint challenging law will continue in the district court.
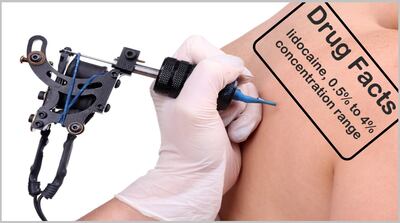
FDA warns six businesses found selling directly to consumers topical formulations containing lidocaine outside the 0.5% to 4% concentration range allowed under OTC monographs for topical analgesics
Agency wants more information about API suppliers as it winds up case against KV Tech for hidden use of Dr. Reddy’s plant and seeks to find disappearing manufacturer of contaminated OTC eye drops.

“I think the symptoms of the core disease of quality is not having an independent layer or just not being able to invest more in quality or whatever the other root causes of addressing these quality issues,” says analytical lab president David Light.

Some advertisers try to avoid scrutiny of regulators and the National Advertising Division when making health-related claims by making a claim “softer.” Annie Ugurlayan, NAD’s assistant director, offers advice on assuring substantiation and a health claim match.