
Recalls

Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.

Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”

Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.

Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.

Amended complaint filed 21 May after US Attorney General in March said the federal and state governments, named as co-plaintiffs, wouldn’t intervene and the court ordered the complaint unsealed. Complaint, initially filed in 2019, is critical of FDA, generally alleging agency failed to conduct sufficient oversight and evaluation of GSK’s testing data for ranitidine.

“I think the symptoms of the core disease of quality is not having an independent layer or just not being able to invest more in quality or whatever the other root causes of addressing these quality issues,” says analytical lab president David Light.
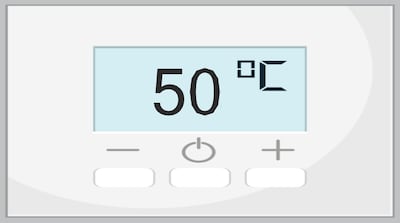
As stability temperature for study of 66 products, it says 50°C is recognized as reasonable temperature product may be exposed to during distribution and handling and is accepted temperature used by pharma industry for accelerated stability studies of at least three months.
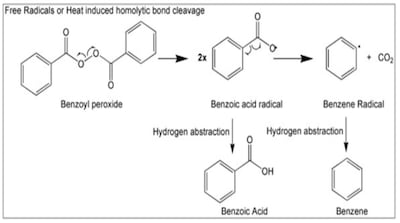
Valisure recommends FDA request recall and halt sales of products containing benzoyl peroxide due to its propensity to form the benzene. Products include numerous OTC monograph topical formulations as well Rx drugs with an acne indication available through approved applications.

Nearly 1,300 rodents exterminated at Arkansas warehouse after FDA investigation found lack of climate control in store of food and OTC drugs requiring dry, room-temperature conditions. Firm pleads guilty in largest monetary criminal penalty in US food safety case.

FDA says FivFivGo, Rebright and South Moon are copycats in packaging easily mistaken for Lumify OTC eye drops. Testing found South Moon contaminated with bacteria linked to an antibiotic-resistant infection; agency warns using any of the three poses risk of eye infection.

Agency proposal, now under HHS review, further reduces chances of losing another whistleblower complaint by taking ORA out of the loop, while proposing a new name for its slimmed-down field organization: Office of Inspections and Investigations. Meanwhile, an agency veteran, Michael Rogers, will lead the revamped organization as associate commissioner.
All lots of nearly 30 different dry eye and lubricating products made by Mumbai-based Kilitch Healthcare India and sold in US by major drug store chains and marketed under multiple private label brands are recalled after FDA investigators found insanitary conditions at firm’s facility.

Since FDA provided draft guidance on quality considerations for ophthalmic drugs, steady stream has continued of OTC brand recalls due to manufacturers’ failure to ensure sterility in production processes and purity of ingredients and other components.

Need for guidance shown not only in recalls over past year of OTC eye drops due to bacterial contaminants found in products and insufficient sterility practices in facilities but also in warning letters sent in 2022 and 2023 to firms manufacturing Rx and OTC ophthalmic drugs.

Recall starts after products across OTC indications, including at-home pregnancy and marijuana tests, “were stored outside of labeled temperature requirements” and “inadvertently shipped to certain stores” in 23 states.

Biomic Sciences recalls all lots of ION* Sinus Support and ION* Biome Sinus after FDE testing found microbial contamination causing reasonable probability of life-threatening adverse events for patients or people who recently underwent nasal or sinus surgery.

Warning letters sent earlier in August note GMP violations for contract manufacturer Denison Pharmaceuticals and customers CalmCo and ALVA-AMCO, which marketed children’s homeopathic products provided by Denison.

Two firms in India, two in South Korea manufacturing toothpastes and hand sanitizers available in US didn’t respond to FDA’s repeated records requests. Six others in those two countries and Canada and Turkey making sanitizers and toothpastes, including products for children, as well as sunscreens were warned about GMP failures after providing records at agency’s request.
Firm reports 3.8% Q1 growth in total nutritional sales to $1.97bn, driven by 19.9% growth in total US sales to $812m as formula and other pediatric product sales grew 36.1% to $465m and adult nutritional sales 3.9% to $353m.

Agency’s latest posting of warning letters submitted also includes more warnings about OTC hand sanitizer production failing to meet GMP requirements.