
Approvals

This week, Medtronic and Hologic announced major safety issues; the US government awarded $110m to innovators in women’s health; CDC partnered with Quest on a bird flu diagnostic; and more.

This week, Hologic announced it would pay $350m for uterine fibroid treatment firm Gynesonics; the US FDA authorized a Novocure cancer treatment and a surgical robot from CMR Surgical; and the IMDRF announced 15 new members.

The US agency hopes that the new program will make information from device clinical trials more accessible to patients, caregivers and providers, including any benefits or risks more likely to occur in certain demographics.

Richard Lowenthal, co-founder and CEO of ARS Pharmaceuticals, highlights the crucial unmet need for needle-free devices to treat type 1 allergic reactions, given challenges associated with current epinephrine injectors. Hear what’s next for the Neffy intranasal spray and ARS Pharmaceuticals.
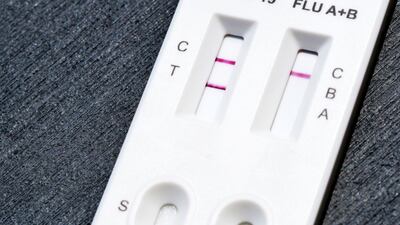
The US Food and Drug Administration has granted a de novo authorization to the Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first over-the-counter flu test to be cleared outside the emergency use pathway.

This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.

This week, Neuralink announced it received US FDA breakthrough device designation for a device to restore sight; medtechs Discure and DeepLook secured new funding; FDA pump recalls from B. Braun Medical and Fresenius Kabi; Axonics prevails in patent infringement lawsuit with Medtronic; Merit Medical buys Cook Medical for $210m.

This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.

Highlights from Medtech Insight's on-the-ground coverage of LSX in Boston.

The US FDA released six more device classifications in early September, including products from Edwards, Interscope, and Baxter Healthcare.

The US FDA has issued a draft guidance document providing recommendations on how device firms can collect patient preference data to share with the agency as it reviews applications. The current draft supersedes final guidance the agency published in 2016.

Sonio says its acquisition by global medical equipment company Samsung Medison is a wrap. Plans for the deal were announced earlier this year.

In this week’s Digital Health Roundup, Medtech Insight’s Ryan Nelson highlights Click Therapeutics’ FDA-cleared digital therapeutics (DTx) for depression and Sinaptica Therapeutics’ personalized neuromodulation for Alzheimer’s patients. Marion Webb discusses her interview with MindMaze’s John Krakauer on their gaming-focused DTx to help people recover from serious brain injuries. Elizabeth Orr introduces new voting members of the new Digital Health Advisory Committee and Natasha Barrow discusses Hello Heart’s new symptom-tracking feature in their heart-focused app.

The devices include a digital therapeutic to treat ADHD and a tongue muscle stimulator to prevent sleep apnea and snoring. They all initially reached market via the de novo process and have now been declared class II.

Renata Medical has received US FDA clearance for its Minima Growth stent, which is designed for neonates, infants and young children and built to expand as the patient ages.

This week, Illumina announced its new CDx has FDA approval; GE and Boston Scientific nabbed CE marks; and the FDA’s Patient Engagement Advisory Committee announced that its October meeting will focus on informed consent in clinical trials.
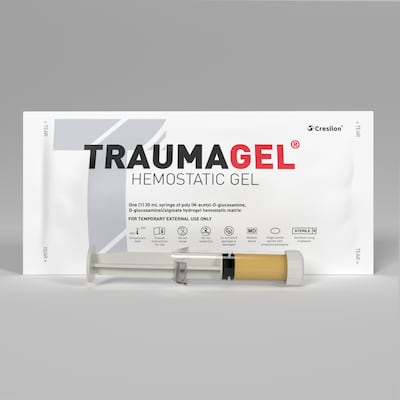
Cresilon CEO Joe Landolina says the newly FDA-cleared Traumagel for moderate and severe bleeding is easier to use than many currently available solutions like gauzes and sponges, and provides faster results.

The US FDA has granted marketing authorization to NOWDiagnostics for the first at-home over-the-counter test to detect syphilis, which the government says is on the rise.

A recent survey of 128 people who had served on CDRH advisory panels found support for several proposals on ways to improve the advisory panel process, including requiring the FDA to explain its reasoning when it departs from panel recommendations.

The FDA designated class II special controls classifications for two diagnostics and force separation catheters.