
Diversity & Inclusion

The US FDA’s Patient Engagement Advisory Committee met Wednesday to discuss ways the agency can empower patients thinking about whether to participate in clinical trials of regulated medical products. Central to the committee’s discussion was not only the type of information patients need to evaluate when considering joining a study, but how that information should be presented to them.

The Australian teletrials program has surpassed expectations at its mid-way point and is being used by drug and device sponsors looking to enhance trial participant recruitment and retention by enabling access to rural, regional and remote areas.

The US FDA has published a discussion paper as part of an effort to ensure all patients have timely access to safe and effective medical devices. The agency says the paper fits into a larger strategy of promoting health equity and is seeking public comment on the initiative.

The E in ESG: SUDs, reuse, disposables, refurbishing, reprocessed devices? Multiple panelists at the 2024 MedTech Forum addressed the role medtech manufacturers should have in the climate change challenge.

A fiscal 2025 report from the US House Appropriations Committee instructs the FDA to halt implementation of its overhaul of LDT regulation. Members also showed concern about overseas device manufacturing and the availability of over-the-counter diagnostics.

In what could amount to a de facto enforcement mechanism, the new draft guidance also “strongly encourages” sponsors to share details about their diversity action plan and enrollment goals with the public.

New draft guidance on clinical trial diversity action plans pushes for disproportionately high enrollment of traditionally underrepresented groups, while also asking companies to tailor global programs to US populations and consider diversity aspects that Congress did not outline.

The US FDA recently announced a new initiative aimed at improving access to cancer trials among traditionally underserved populations. myTomorrows CEO Michel van Harten talked to Medtech Insight about how the agency’s plan might reshape the landscape of clinical trials going forward.

To avoid a wider health equity gap for home-use devices, companies should make social determinants of health a key consideration in device development, former nurse Amy Hester says.

As a biomedical engineer, Blair Hirst saw how health technology could help patients – and how some communities were slowest to benefit. As founder of the Digital Health Review, she now works to improve equity in health care spaces.

The new tool provides a way of quantifying the degree of diversity by race/ethnicity, sex and age in clinical trials, but the results will not factor into the US health technology assessment body’s cost effectiveness determinations for new drugs.

A study of diabetes rates across the US over four years reveals significant increases in the disease in many states. Tobias Oerum, diabetes advocate and cofounder of the company that conducted the study, discussed the data and some of the factors contributing to this troubling trend with Medtech Insight.

US FDA oncology officials are concerned that the entities sponsors are consulting in developing and implementing clinical trial diversity plans are not sufficiently diverse themselves and do not represent patients in underserved communities.

BD CTO Beth McCombs discusses her role in driving innovation, including the integration of AI and generative AI to create efficiencies, and passion for creating a culture of inclusion, diversity and equity.

President Joe Biden signed an executive order the White House said represents the most comprehensive executive action to date on improving women’s health. The move follows the president’s call during his State of the Union for Congress to invest $12bn in women’s health research.

Marissa Fayer is dedicated to advancing women’s health. The founder and CEO of HERhealthEQ, a global nonprofit focused on women’s health equity in emerging and developing countries, Fayer is also the CEO of DeepLook Medical. As part of our focus on women’s health in honor of Women’s History Month, Fayer spoke to Medtech Insight about the importance of having more women in all sectors of medtech, both for the benefit of industry as well as women patients.
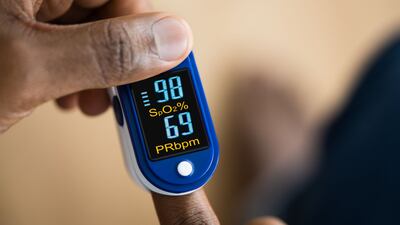
A 15-month UK independent review of ethnic and other biases in device design has called for treatment inequity to be addressed at the highest levels of government, more resources for the MHRA and equity assessments to be included in approvals and post market surveillance.

This week, the FDA reminded consumers that smart watches and rings are not blood glucose monitors; First Lady Jill Biden announced a $100M women’s health initiative; and the Zimmer ROSA Shoulder System got FDA clearance.

In a recent guidance document, the FDA explains how trial sponsors should collect and present data on the race and ethnicity of trial participants.

Sam Ajizian, chief medical officer of Medtronic’s patient monitoring operating unit, spoke to Medtech Insight about his reaction to a recent FDA panel meeting on ways to improve pulse oximetry accuracy and equity.