

This week, HistoSonics announced it will bring its ultrasound system for destroying liver tumors into VA hospitals, Edwards Lifesciences reports encouraging TRISCEND II trial results at TCT, study finds blood test for CRC screening are less cost-effective than alternatives, and more.

Medtech Insight was on the ground at HLTH looking for innovative companies. Learn about seven start-ups using AI to help physicians detect conditions including prostate and breast cancers, seizures and heart failure; assess patients for cognitive decline validate and deploy algorithms, and monitor patients in and out of the hospital.

This week, Hologic announced it would pay $350m for uterine fibroid treatment firm Gynesonics; the US FDA authorized a Novocure cancer treatment and a surgical robot from CMR Surgical; and the IMDRF announced 15 new members.
Tricuspid valve innovation has taken off since the US FDA’s 2023 authorizations of Edwards' Evoque and Abbott's TriClip systems. Whether to repair or replace tricuspid valves remains an open, nuanced question among cardiologists. Dr. Henrik Treede of University Hospital Mainz and TriCares CEO Ahmed Elmouelhi offer views on the evolving space.

The Octane Medical Innovation Forum brought together industry experts, entrepreneurs and investors to discuss a range of topics. Medtech Insight was on the ground to bring some memorable perspectives from industry leaders.

This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.
Spain-based Inbrain Neuroelectronics plans first-in-human study to show safety of its graphene-based technology in direct contact with human brain while also developing a second interface for treating Parkinson’s disease.
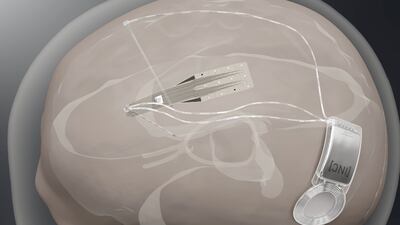
After announcing positive results showing that its Stentrode BCI is safe in six patients, brain-computer interface company Synchron is planning a pivotal trial to eventually file for FDA approval.

Tim Schmid, executive VP and worldwide chairman of J&J MedTech, expects Shockwave, acquired in April, and Abiomed, purchased in late 2022, to be “long-term gems” for the company. Cardiovascular is among higher-growth segments where J&J has concentrated investments in recent years, along with robotic surgical systems.
According to Zacks Equity Research, the new trial seeks to support AlphaVac’s adoption in the European market, where pulmonary embolism prevalence and severity is an estimated 435,000 events annually.

Medtronic chairman and CEO Geoff Martha addressed the promise of artificial intelligence in the company’s medical technologies, AI monetization opportunities, and risk of AI overregulation in a 4 September session at the Wells Fargo Healthcare Conference.

Highlights from Medtech Insight's on-the-ground coverage of LSX in Boston.

Smiths Medical has recalled scores of Bivona tracheostomy tubes due to a manufacturing defect that can result in disastrous consequences. The company reports multiple injuries, and one death, linked to the devices.

The US FDA released six more device classifications in early September, including products from Edwards, Interscope, and Baxter Healthcare.

This week, a Delaware court awarded Auris Health shareholders $1bn in a lawsuit against Johnson & Johnson; Abbott recalled some FreeStyle Libre 3 sensors; and McKesson purchased a controlling interest in a Florida cancer care chain.
The US Food and Drug Administration released six warning letters and two close-outs last month, including four warnings to marketers of unauthorized continuous positive airway pressure (CPAP) cleaners or sanitizers.

In this week’s Digital Health Roundup, Medtech Insight’s Ryan Nelson highlights Click Therapeutics’ FDA-cleared digital therapeutics (DTx) for depression and Sinaptica Therapeutics’ personalized neuromodulation for Alzheimer’s patients. Marion Webb discusses her interview with MindMaze’s John Krakauer on their gaming-focused DTx to help people recover from serious brain injuries. Elizabeth Orr introduces new voting members of the new Digital Health Advisory Committee and Natasha Barrow discusses Hello Heart’s new symptom-tracking feature in their heart-focused app.

Renata Medical has received US FDA clearance for its Minima Growth stent, which is designed for neonates, infants and young children and built to expand as the patient ages.

Inari Medical is updating use instructions for a clot-removing catheter due to the potential for serious adverse effects, including death.

This week, a medical group sued the FDA to block a lab-developed test rule; the FDA published guidance on device classifications; Defibtec issued a recall of its chest compression device and ICU Medical updated instructions for its infusion pump batteries; Maui Imaging raised a $4m DOD grant to put imaging tech into military-based trauma units.