
Complete Response Letters

The company will file a new drug application with the US FDA by the end of the year for tradipitant in a different indication, motion sickness.

Sponsor of psychedelic PTSD treatment will try rarely successful formal dispute resolution process after receiving a complete response letter.

US FDA would need to approve 44 novel agents by year-end to match 2023’s big total, but only 40 candidates are known to have user fee goals in the second half of 2024.

A Pink Sheet analysis finds US approval preceded European Union clearance for 80% of products approved in both areas, but when EU approval came first, it beat the FDA by a median of 13 months. US-first approvals came close to six months before the EU.

Rocket Pharmaceuticals’ gene therapy Kresladi, Daiichi Sankyo/Merck’s patritumab deruxtecan, and AbbVie’s foscarbidopa/foslevodopa (ABBV-951) received CRLs because of manufacturing concerns.
In a year full of regulatory milestones for the firm, PTC Therapeutics hopes to set some approval precedents – and basically hopes its candidates have an easier time of it than they have had before.

Vanda CEO tells the Pink Sheet that the firm is going straight to appellate court to refute agency’s rejection of jet lag indication for Hetlioz. Similar battles may lie ahead over Vanda’s applications on Hetlioz for insomnia and tradipitant for gastroparesis.

Crowded calendar could bring to an end the slow start for novel approvals in 2024, as decisions come due for Lilly’s donanemab, Merck’s sotatercept, Regeneron’s odronextamab and more.

US FDA’s drugs center posted one of its lowest monthly approval counts of original applications in January, but new indications filled the gap, including new claims for Merck’s Keytruda, Takeda immunoglobulins, and Sanofi/Regeneron’s Dupixent.

After a US FDA site inspection raised questions about the contract manufacturer of the potential first-in-class Claudin 18.2 inhibitor that could not be answered by the original January PDUFA date, Astellas will need to refile the application, but has not committed to when that might be.

US FDA’s biologics center posted a historic 17 novel approvals, blowing past previous records, while the drug center’s 55 novel agents was close to its 21st century high.

Aldeyra’s bid to rely on a single Phase III trial after an initial one failed is rejected by FDA, but firm already has a special protocol assessment under review for a trial that could provide the symptom data it says agency needs to approve the RASP modulator in dry eye.

Safety signals were the most common cause of discontinued candidates in the breakthrough therapy designation program in 2023.

Pink Sheet infographic breaks down the breakthrough therapy designations made by the US FDA, with statistics on what’s been approved, rescinded, and everything in between.

Pink Sheet reporters and editor discuss the problems that emerged for the US FDA from just the threat of a government shutdown, take-aways on the FDA advisory committee process based on the complete response letter issued for Alnylam’s Onpattro, and incentives that the FDA could offer for ARPA-H projects that reach the application stage.

In going against an advisory committee vote, the agency produces ironic echoes with the history of the approved treatment for ATTR-CM, Pfizer’s Vyndaqel.

The situation also is a perfect example of FDA doing what it says it does – paying more attention to the thinking behind advisory committee votes than the votes and vote totals themselves.

Fourteen user fee goal dates are on the October calendar, including three novel agents, according to the Pink Sheet US FDA Performance Tracker.

CDER reviewers don’t buy that safety concerns with exenatide implant ITCA 650 are in line with the risks labeled for already approved GLP-1 agonists for type 2 diabetes – even other formulations of the same drug – potentially due to unique considerations associated with Intarcia’s delivery method.
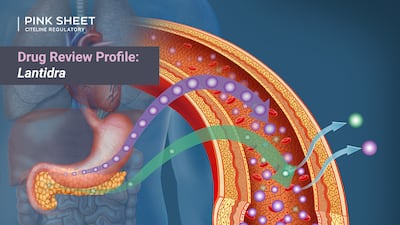
Although the CRL delayed the cell therapy’s approval by almost two years, additional CMC-related testing requested by the FDA ultimately came to be seen as a good thing by sponsor CellTrans because it provided assurance of product consistency.