United Kingdom

The Life Sciences Innovative Manufacturing Fund, set out in the UK budget last month, is live for applications. More UK medtechs would benefit if the cost threshold were scaled down, the industry argues.

The public alongside clinicians and industry experts is asked to submit ideas for reforming the NHS via a dedicated online platform ahead of the new NHS Plan in 2025.

The Design for Life roadmap will help medtech companies comply with the UK NHS’s Net Zero 2045 greenhouse gas emissions target. A dedicated medtech innovation center is mooted.

A new UK medtech survey sets out industry’s market access and regulatory concerns and makes clear where system users see the need for improvement. There are some grounds for optimism, but the MHRA’s planned rise in regulatory fees could undo some of the good work.

Artificial intelligence and digital in healthcare are among four key scientific development areas that will benefit from the support of the UK Regulatory Innovation Office, the launch of which was announced by the government on 8 October.
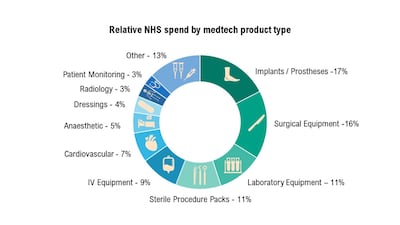
Innovation in life sciences and UK NHS adoption of technology are high on UK’s political agenda in the post-election period. Projects at the MedTech Directorate and NICE, and support from the Office for Life Sciences, are playing into a renewed sense of optimism.

No longer a watchdog, the UK MHRA wants to be seen as an enabling regulator, using new methodologies and data sources to bring device innovations safely into use, says agency chief executive June Raine.
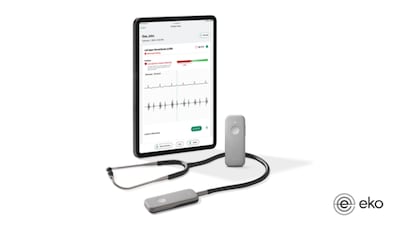
The results from a trio of studies show that AI-powered digital stethoscopes are effective at identifying patients at elevated risk of experiencing heart attacks and other major cardiac adverse events, according to Eko Health, whose technology was used to screen the participants in the studies.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.

The ISRCTN clinical trials registry has launched an improved dashboard to provide metrics that reveal how many studies are complying with key transparency requirements. Badges are in place for individual studies meeting the transparency criteria.

Those working in the medtech sector returned from their traditional August break to a growing sense that the EU is more collectively aligned about the need to improve the medtech regulations and soon.

The UK medical devices regulator has announced a consultation on a pre-market statutory instrument to be completed by the end of 2024.

Philip Morris described a scenario of being essentially blacklisted by the health care sector and its CDMO partners as it threw in the towel on its £1.1bn acquisition of UK inhalation specialist Vectura. It agreed to offload the firm at a fraction of the initial cost.

The NHS England and NICE “rules-based pathway” plan for medtech innovation adoption includes a proposed funding cap that could stymie its aims. Elsewhere, progress is reported on the MHRA’s AI Airlock and UK post-market surveillance.

UK-based Biocomposites will begin selling in the UK its next-generation osteoinductive bone graft substitute, NanoBone, which came with its acquisition of Artoss GmbH in June 2023. Meanwhile, the company has purchased remaining shares in the manufacturers of SYNICEM and Subiton antibiotic bone cements and preformed antibiotic-loaded spacers.

The firm’s dual-chamber leadless pacemaker system with is expected to be a growth driver for Abbott and already has acquired approximately 30%-40% of market share from Medtronic since its approval by the US FDA in July 2023, according to GlobalData.

Can the new UK government's raw enthusiasm for NHS reform be a catalyst for real change where countless past attempts to address the national provider's shortcomings have failed to hit the spot? And what might the 10-year plan mean for medtech?

The UK MHRA has given medtech companies and stakeholders seven weeks to comment on proposed use fee rises for 2025. 16% rises are mooted for a variety of services.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 80 documents have been posted on the tracker since its last update.
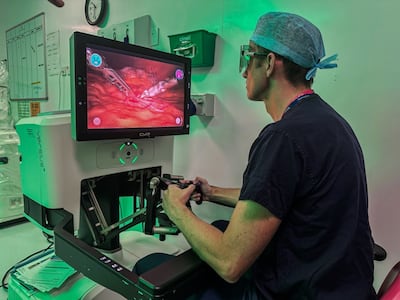
CMR Surgical has launched a multicenter pediatric clinical trial for its Versius surgical robot system across three UK sites. The trial will enroll 150 children for urological procedures, with patient outcomes followed for up to a year post-surgery.