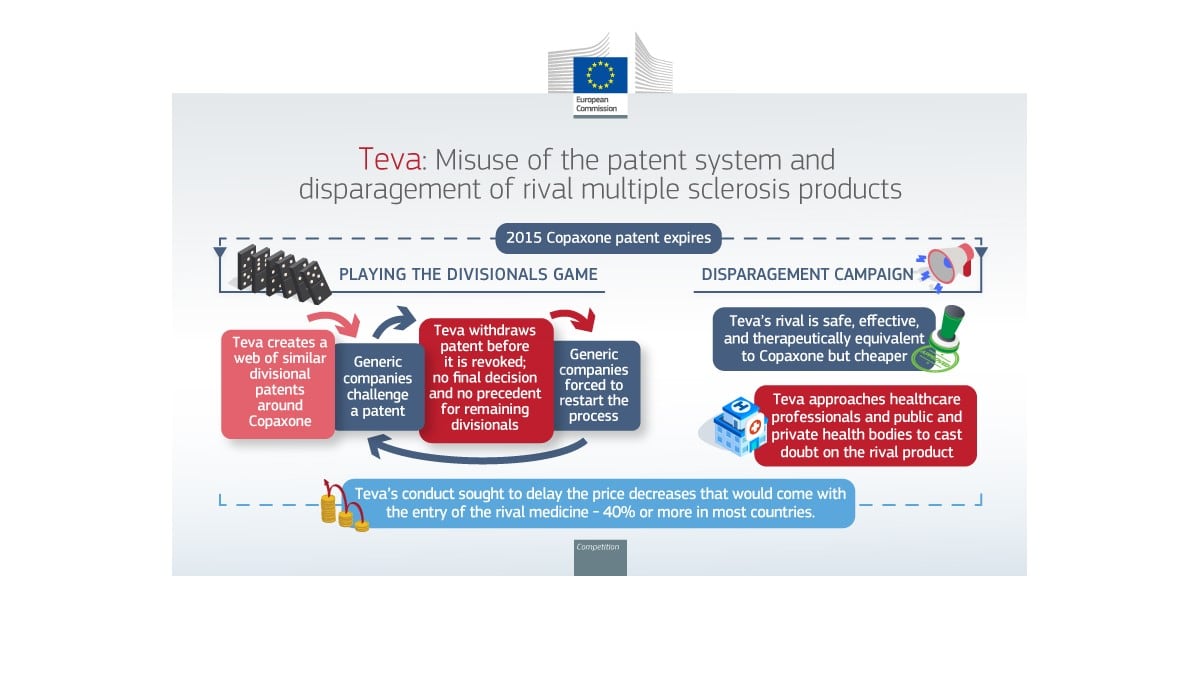
Rare Diseases: New Technologies Outpacing Regulatory Pathways In UK
A prominent rare disease researcher has praised the UK’s Department for Health and Social Care for its transparency and willingness to work with experts, but said that regulatory systems needed to speed up to facilitate patient access to innovative treatments.