ADVERTISEMENT
Enforcement
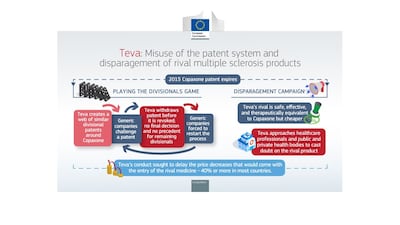
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Trade group ready to work hand-in-hand with agency and other supplement industry stakeholders on potential regulatory changes or improvements, says president and CEO Steve Mister. “None of them are upsetting the basic balance of things that DSHEA was attempting to do, but there are things with 30 years that we've identified that need to be kind of fixed.”

Teva resolved two US Department of Justice civil suits accusing the firm of violating the US Anti-Kickback Statute and the False Claims Act by allegedly conspiring to fix the price of three generic drugs and paying Medicare patients’ copays for its multiple sclerosis brand product Copaxone.

Teva has resolved a pair of civil US Department of Justice lawsuits accusing the firm of violating the US Anti-Kickback Statute and the False Claims Act through its alleged conduct conspiring to fix the price of three generic drugs and for allegedly paying Medicare patients’ copays for its multiple sclerosis brand Copaxone.

The owner of a Chicago COVID-19 testing lab plead guilty to wire fraud for billing the government for COVID-19 tests that were not performed. Also, test developer Talis Biomedical agreed to pay $32.5m to settle a shareholder suit.

The Federal Trade Commission’s Consumer Protection head Sam Levine says bipartisan leaders at the state and federal levels are taking on consumer protection issues like never before and using the FTC’s approach as a model for legislation.
Although the FTC’s administrative complaint focuses only on insulins, the commission hopes it will have a broader impact by driving reforms of rebate and contracting practices for other drugs as well.

Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.

Reorganization creating in Human Foods Program makes the work of additional staff available for dietary supplement office programs. FDA’s “got a little tiny workforce” for “a huge industry,” says Commissioner Robert Califf.