ADVERTISEMENT
Compliance
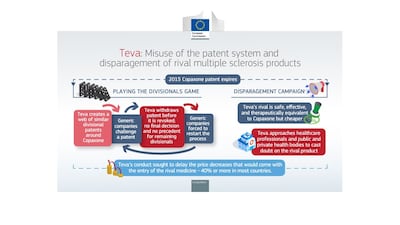
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Trade group ready to work hand-in-hand with agency and other supplement industry stakeholders on potential regulatory changes or improvements, says president and CEO Steve Mister. “None of them are upsetting the basic balance of things that DSHEA was attempting to do, but there are things with 30 years that we've identified that need to be kind of fixed.”

The Design for Life roadmap will help medtech companies comply with the UK NHS’s Net Zero 2045 greenhouse gas emissions target. A dedicated medtech innovation center is mooted.

Requests for “enforcement actions are not within the scope of FDA’s citizen petition procedures,” CDER says, rejecting petition dosing device firm Parenteral Technologies submitted as it prepares for workshop on Pediatric Research Equity Act requirements for OTC NDA sponsors.

The European Parliament intends to send an official message to the European Commission to try and push for an early revision of the Medical Device Regulation.

The European Commission’s proposed timeframe for revising the MDR and IVDR is far too long, Parliament believes, and is resulting in increasingly outdated products in healthcare establishments around European threatening patient care.

UK pharma also reaches agreement in principle, subject to DoJ approval, to pay $70m to resolve a whistleblower complaint filed by Valisure, the testing lab which in 2019 raised concerns about a potential link between the use of drugs containing ranitidine, a histamine-2 blocker, and cancer.

NDAs for additional OTC products containing acetaminophen and/or NSAIDs and indicated for use by children between 2 and less than 12 years old would trigger compliance by application sponsors with the act passed in 2003 to address lack of pediatric use information in drug labeling.

Having 50 notified bodies under the Medical Device Regulation is a landmark achievement for the EU after a long and slow, journey to reach this point.