ADVERTISEMENT
Europe
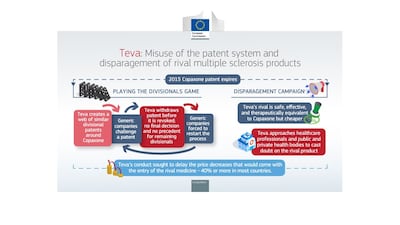
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

The European Medicines Agency is conducting a public consultation on proposed revisions to its policy on how it handles any conflicts of interest of its scientific committee members and experts.

Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

While Roche admits that an acceleration of biosimilar competition to its blockbuster IL-6 inhibitor Actemra/RoActemra is anticipated in the final three months of the year, the Swiss originator has been surprised by the lack of challenge to its franchise so far.

In the wake of its recent acquisition of dermatology assets from Canute Pharma, UK-based Aspire Pharma is looking to new frontiers of expansion in Europe and beyond. Chief executive Richard Condon talks to Generics Bulletin about the next steps on the niche generics and value added medicines firm’s journey.

With a looming deadline of 1 January 2025 for firms supplying Northern Ireland to comply with the Windsor Framework, UK generics and biosimilars association the BGMA has warned of potential supply interruptions due to requirements that include a “UK only” label for all packs as well as UK-based batch testing for biologicals.

Olivér Várhelyi’s plans for his new role boil down to continuing legislative projects and other initiatives that have already been set in motion.

The updated directive could risk the supply of critical medicines and fail to incentivize greener product development more generally, warns European industry group EFPIA.

A ruling by the Court of Justice of the EU has produced a clear definition of what constitutes the “first” marketing authorization when companies apply for SPCs on pharmaceutical products.

The Pink Sheet highlights recent comments and insights from pharma officials and executives on key issues we are covering.