ADVERTISEMENT
Generic Drugs
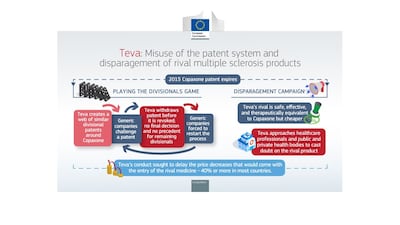
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Sponsors of generic drug applications that miss a goal date, but do not receive an action because of complex scientific or legal questions, would get a notice outlining the lingering issue as part of a new pilot program that might become permanent in the next review cycle.

Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

In the wake of its recent acquisition of dermatology assets from Canute Pharma, UK-based Aspire Pharma is looking to new frontiers of expansion in Europe and beyond. Chief executive Richard Condon talks to Generics Bulletin about the next steps on the niche generics and value added medicines firm’s journey.

While the firm’s mind and money continues to gear towards branded and novel drugs, India’s Sun Pharma still maintains a mammoth global generics business, including in the US where Sun saw a healthy leap in sales during its financial second quarter.
After sharing multiple updates to the regulatory status of its ketamine formulation, PharmaTher received a complete response letter from the FDA, which required minor information and clarifications from the company.

Generics of the schizophrenia treatment Latuda generated more than $4bn in savings a year after their 2022 approval, according to an FDA study.
French industry association Gemme has issued a stark warning that recent legislative proposals – including a revision to the country’s clawback mechanism and steeper financial penalties for failing to hold sufficient safety stocks – will “bring about the end of the generic economic model in France.”

Years after the patent application’s initial filing in 2007, the Indian Patent Office has dismissed ViiV’s claims in response to multiple opposition filings launched against the pharma player.

With a looming deadline of 1 January 2025 for firms supplying Northern Ireland to comply with the Windsor Framework, UK generics and biosimilars association the BGMA has warned of potential supply interruptions due to requirements that include a “UK only” label for all packs as well as UK-based batch testing for biologicals.