ADVERTISEMENT
BioPharmaceutical

The company, now the most advanced clinically in the psychedelic space after the rejection of Lykos's MDMA-based post-traumatic stress disorder drug, is cutting its workforce by a third and narrowing its research focus after shifting the timeline for its late-stage depression candidate.
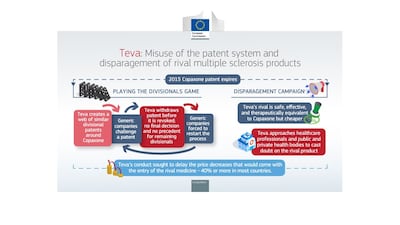
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Recent moves in the industry include changes at the top at Fennec Pharmaceuticals, plus Opthea Acquires Chief Financial Officer From Amarin, and Disc Medicine gets a new chief technical officer.

Only days after its initial announcement, Hypera Pharma has refused EMS’ proposed merger on grounds of undervaluation and different strategic priorities.

Sponsors of generic drug applications that miss a goal date, but do not receive an action because of complex scientific or legal questions, would get a notice outlining the lingering issue as part of a new pilot program that might become permanent in the next review cycle.

Respondents to the FDA’s questions over biosimilar development did not hold back. So, what is it: product-specific or product class-specific guidance? Or nothing?

The UK group began more clinical trials last year than any other company, a new report from Citeline has found.

Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

In a citizen petition to the FDA, Alvotech has called on the US agency to refrain from approving as interchangeable certain Stelara biosimilars that use a different cell line to its own ustekinumab product – including the Pyzchiva version set to be brought to market by Samsung Bioepis and Sandoz.

As it posts third-quarter numbers that again reveal the decline of revenues from its multiple sclerosis portfolio, the US biotech major is entering into the hot area of targeted protein degradation to boost its immunology and neurology pipeline.