ADVERTISEMENT
Japan

While Roche admits that an acceleration of biosimilar competition to its blockbuster IL-6 inhibitor Actemra/RoActemra is anticipated in the final three months of the year, the Swiss originator has been surprised by the lack of challenge to its franchise so far.

Only a month after filing their second biosimilar application with Japanese regulators, Alvotech and Fuji have submitted a further one for an undisclosed biosimilar as part of their local partnership.

Stay up to date on regulatory guidelines from around the world with the Pink Sheet's Guidance Tracker. The complete Global Pharma Guidance Tracker, with sortable and searchable listings going back to 2014, is available online.

Following consideration by our independent expert judging panel, the final shortlist of entries has now been revealed for the Citeline Japan Awards 2024. Join us at the event in Tokyo on 22 October!

Japan has started to charge patients a portion of the difference between the reimbursement price of the generic and non-generic product if they insist on the latter without a supporting recommendation from the prescribing physician, in a policy designed to further drive generic use.

Japan has started to charge patients a portion of the difference between the reimbursement price of the generic and non-generic product if they insist on the latter without a supporting recommendation from the prescribing physician, in a policy designed to further drive generic use.

CEO Chris Cargill talks to Scrip about Sosei Heptares’ new identity as Nxera, the ups and downs of being big pharma’s go-to small-molecule drug hunter and its move into commercialization in Japan.
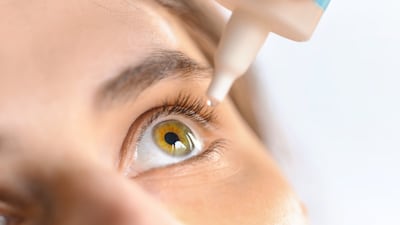
The dual agonist of the FP and EP3 receptors has shown efficacy in a Japanese Phase III trial and completed its US Phase II study, which aligns with the Japanese firm’s global expansion strategy for glaucoma products.

Japan plans to offer wider support to foreign firms and ventures with innovative candidates to start clinical trials in the country, as part of key measures from current prime minister Fumio Kishida.

Taisho’s in-house insomnia therapeutic candidate vornorexant offers a potential advantage of a shorter half-life compared to its competitors, which can contribute to patients’ QOL.