Digital Technologies

This week, HistoSonics announced it will bring its ultrasound system for destroying liver tumors into VA hospitals, Edwards Lifesciences reports encouraging TRISCEND II trial results at TCT, study finds blood test for CRC screening are less cost-effective than alternatives, and more.

Medtech Insight talked with GE HealthCare’s chief AI officer Parminder “Parry” Bhatia at HLTH about the firm’s new CareIntellect for Oncology offering to help clinicians make efficient use of multimodal patient data, his vision for projects within AI Innovation Lab, and the future of AI in health care.

The US FDA’s Patient Engagement Advisory Committee met Wednesday to discuss ways the agency can empower patients thinking about whether to participate in clinical trials of regulated medical products. Central to the committee’s discussion was not only the type of information patients need to evaluate when considering joining a study, but how that information should be presented to them.

Recognizing October as cybersecurity awareness month, the US FDA has added new publications to its list of resources concerning the cybersecurity of medical devices.

At HLTH 2024, GE HealthCare unveiled CareIntellect for Oncology, a generative AI-driven platform to help oncologists quickly assess patient data across multiple sources to save time and improve treatment planning. The platform, currently in evaluation at two US hospitals, is set for launch in the second half of 2025.

Medtech Insight was on the ground at HLTH looking for innovative companies. Learn about seven start-ups using AI to help physicians detect conditions including prostate and breast cancers, seizures and heart failure; assess patients for cognitive decline validate and deploy algorithms, and monitor patients in and out of the hospital.

This week, Medtronic and Hologic announced major safety issues; the US government awarded $110m to innovators in women’s health; CDC partnered with Quest on a bird flu diagnostic; and more.

The co-founder of Pure Global discusses the regulatory consultancy's use of AI to support clients’ marketing submissions and other needs, as well as the AI-enabled medtech landscape and opportunities in China and Southeast Asia for accessing patient data for AI development purposes.

‘Unicorn’ Startup Owkin On EU’s AI Act, Funding Needs: ‘Europe Doesn’t Want To Be Under-Competitive’
The EU Artificial Intelligence Act, the first official AI regulation globally, entered into force on 1 August 2024. Two months on, Medtech Insight sits down with AI diagnostic startup Owkin’s chief diagnostic officer Meriem Sefta, and senior vice president of public affairs and impact Yedidia Levy-Zauberman, to discuss how the company is adapting to the EU AI Act, the opportunity it presents, and what is needed next for AI deployment in the EU.

Deloitte Center for Health Solutions surveyed 85 leaders from medtech organizations for its report, “Is Generative AI changing the game for medtech?” Respondents replied overwhelmingly “yes,” while acknowledging the industry thus far has only scratched the surface of AI’s potential.

Venture capital investment in healthtech is showing signs of early recovery in the first half of 2024 after the long hangover of a downturn that followed booming investments during the pandemic years, according to a new report from Silicon Valley Bank (SVB). Despite ongoing challenges, healthtech is on a positive trajectory and hovers between $3.5bn and $4.5bn per quarter, surpassing pre-pandemic levels.

Start-ups pitched a diverse deck of innovative technologies to three judges and an audience of potential investors, strategics and physicians at the Octane Medical Innovation Forum in Irvine, CA. Highlights include neuromodulation company Sinaptica Therapeutics, which won the competition for both “People’s Choice” and “Judge’s Choice.”

Industry experts believe the future of medtech will be centered around patients and their data. To thrive, medtech companies must integrate themselves more organically in the patient continuum and adopt more of a Big Tech mindset in order to meet users' evolving expectations.

Marabio Systems says the new funding will help accelerate efforts to bring a blood test to market in 2025 that will accurately determine if a mother is a carrier of antibodies that cause MARA, a subtype of autism that believe to cause more severe behavior.

The Octane Medical Innovation Forum brought together industry experts, entrepreneurs and investors to discuss a range of topics. Medtech Insight was on the ground to bring some memorable perspectives from industry leaders.

The US FDA’s Office of Women’s Health provides a research roadmap to address health concerns specific to women. The FDA recently updated the roadmap, outlining areas in which further research is needed.

This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.
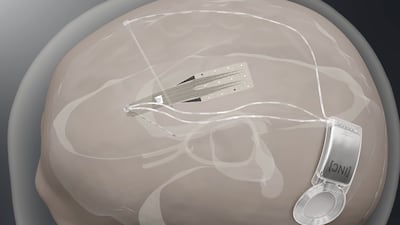
Spain-based Inbrain Neuroelectronics plans first-in-human study to show safety of its graphene-based technology in direct contact with human brain while also developing a second interface for treating Parkinson’s disease.
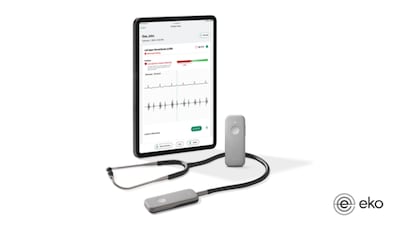
The results from a trio of studies show that AI-powered digital stethoscopes are effective at identifying patients at elevated risk of experiencing heart attacks and other major cardiac adverse events, according to Eko Health, whose technology was used to screen the participants in the studies.

Altoida CEO Mark Jones has high hopes that the company’s digital assessment tool will be approved by the FDA to be used along with blood biomarker testing by primary care doctors to help predict Alzheimer’s disease before patients show symptoms.