Strategy

At HLTH 2024, GE HealthCare unveiled CareIntellect for Oncology, a generative AI-driven platform to help oncologists quickly assess patient data across multiple sources to save time and improve treatment planning. The platform, currently in evaluation at two US hospitals, is set for launch in the second half of 2025.

Despite questions surrounding the SEC rule, including disputes being litigated in the US Eighth Circuit, companies must prepare to meet the new climate disclosure requirements in addition to related mandates in California and abroad. Experts emphasize opportunities beyond compliance.
Craig Eagle, chief medical officer of Guardant Health, provides his view on an increasingly competitive liquid biopsy market in this interview with Medtech Insight.

‘Unicorn’ Startup Owkin On EU’s AI Act, Funding Needs: ‘Europe Doesn’t Want To Be Under-Competitive’
The EU Artificial Intelligence Act, the first official AI regulation globally, entered into force on 1 August 2024. Two months on, Medtech Insight sits down with AI diagnostic startup Owkin’s chief diagnostic officer Meriem Sefta, and senior vice president of public affairs and impact Yedidia Levy-Zauberman, to discuss how the company is adapting to the EU AI Act, the opportunity it presents, and what is needed next for AI deployment in the EU.
Abbott’s Diabetes Care Division has recorded meteoric growth over recent years. Partnerships and a laser-focused strategy for driving patient access are at the core of its success, says Chris Scoggins, SVP of commercial operations and marketing.

At this year’s LSX Nordic conference, experts from Baxter, Philips and Novo Nordisk described key attributes they look for when selecting companies for partnerships or acquisitions and provided advice to founders on how and when to start positioning their start-ups for such opportunities.

Industry experts believe the future of medtech will be centered around patients and their data. To thrive, medtech companies must integrate themselves more organically in the patient continuum and adopt more of a Big Tech mindset in order to meet users' evolving expectations.

The Octane Medical Innovation Forum brought together industry experts, entrepreneurs and investors to discuss a range of topics. Medtech Insight was on the ground to bring some memorable perspectives from industry leaders.

This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.

This week, Neuralink announced it received US FDA breakthrough device designation for a device to restore sight; medtechs Discure and DeepLook secured new funding; FDA pump recalls from B. Braun Medical and Fresenius Kabi; Axonics prevails in patent infringement lawsuit with Medtronic; Merit Medical buys Cook Medical for $210m.

There is considerable focus at present on how far notified bodies can go in helping position their clients to have as smooth a conformity assessment passage as possible. It is a fine balancing act for all involved, but a new document gives useful insights.

Highlights from Medtech Insight's on-the-ground coverage of LSX in Boston.

Clue Plus members will have access to 50% off a Headspace subscription for tailored guidance, support and validation to help manage cycle-related stress and improve overall well-being.
Edwards Lifesciences’ Critical Care business “invented the hemodynamic monitoring category, and its solutions are currently used in more than 10,000 hospitals globally to better understand the cardiovascular condition in real-time for critically ill patients, which helps improve outcomes,” says BD, which sees synergies and new innovation opportunities across the groups’ data sets and platforms. Acquired for $4.2bn cash, Critical Care generated more than $900m in revenue in 2023.

During a tour of Truvian’s San Diego headquarters, Medtech Insight spoke with the company’s top executives about their unique three-in-one blood-testing benchtop system, plans for FDA regulatory filing, and marketing strategy.

This week, a medical group sued the FDA to block a lab-developed test rule; the FDA published guidance on device classifications; Defibtec issued a recall of its chest compression device and ICU Medical updated instructions for its infusion pump batteries; Maui Imaging raised a $4m DOD grant to put imaging tech into military-based trauma units.

Getinge says it plans to pay about $477m for organ transport and services company Paragonix Technologies in an effort to “redefine the market standard in transplantation.”
Moximed aims to accelerate adoption of its MISHA Knee System, De Novo-approved by the US FDA in April 2023, with $91m in Series D funding. Chris Gleason, president and chief executive officer, offers perspective on the company’s innovative technology and commercial growth activities.

Announced in conjunction with a $97m Series D financing round, Neptune underscores its gastrointestinal focus and robotics aspirations by spinning out Jupiter Endovascular, which will leverage $21m of the funding to support ongoing development of its Endoportal Control technology.
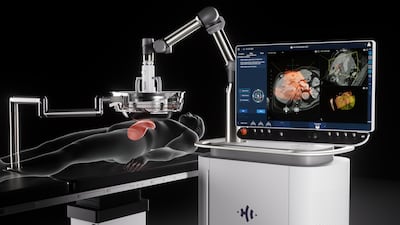
Following FDA clearance of its Edison Histotripsy System in October 2023 for the non-invasive destruction of liver tumors using focused ultrasound, HistoSonics announces plans for $102m in new funding led by Alpha Wave Ventures. “Bubbles have never been so powerful,” the company says.