
This week, HistoSonics announced it will bring its ultrasound system for destroying liver tumors into VA hospitals, Edwards Lifesciences reports encouraging TRISCEND II trial results at TCT, study finds blood test for CRC screening are less cost-effective than alternatives, and more.

Medtech Insight talked with GE HealthCare’s chief AI officer Parminder “Parry” Bhatia at HLTH about the firm’s new CareIntellect for Oncology offering to help clinicians make efficient use of multimodal patient data, his vision for projects within AI Innovation Lab, and the future of AI in health care.

House Republicans say the FDA has not done enough to support its laboratory safety office, despite past recommendations.

Oxford Medical Products (OMP) announced positive results from its first randomized controlled trial of weight loss device Sirona, an inert dual-polymer hydrogel pill that expands in the stomach to mechanically suppress appetite. CEO Camilla Easter views the device as complementary and potentially synergistic with GLP-1 receptor agonists “at a fraction of the cost.”

Medtech Insight spoke with Vicky Demas, CEO of Identifeye, about plans for bringing the company’s AI-powered retinal screening system for early detection of diabetic retinopathy to primary care facilities. More than 50% of the roughly 38 million Americans who have diabetes skip retinal screenings at present, increasing risk of developing the leading cause of blindness in adults.
The medical device industry supports the FDA's draft guidance document on Diversity Action Plans but seeks flexibility and clarity, especially for international and IVD trials, and recommends using real-world data for postmarket studies.

During the Medtech Conference in Toronto, three of the industry’s leading CEOs shared their insights into the rapidly changing landscape of health care and how the latest advancements have the potential to make life better for patients everywhere.

Start-ups pitched a diverse deck of innovative technologies to three judges and an audience of potential investors, strategics and physicians at the Octane Medical Innovation Forum in Irvine, CA. Highlights include neuromodulation company Sinaptica Therapeutics, which won the competition for both “People’s Choice” and “Judge’s Choice.”
Slow adoption of alternatives to animal testing in the current decentralized regulatory framework shows the need for a ‘one-stop shop’ at FDA that can provide advice, precedents and qualification programs.

This week, J&J announced that it was buying heart failure device firm V-Wave; Procept got the FDA’s OK on a clinical trial of its Aquablation treatment for prostate cancer; and CMS began to consider Medicare reimbursement of Abbott’s TriClip tricuspid repair device.

Marabio Systems says the new funding will help accelerate efforts to bring a blood test to market in 2025 that will accurately determine if a mother is a carrier of antibodies that cause MARA, a subtype of autism that believe to cause more severe behavior.

The Octane Medical Innovation Forum brought together industry experts, entrepreneurs and investors to discuss a range of topics. Medtech Insight was on the ground to bring some memorable perspectives from industry leaders.

The US FDA’s Office of Women’s Health provides a research roadmap to address health concerns specific to women. The FDA recently updated the roadmap, outlining areas in which further research is needed.

Patrick Alexandre, Crossject CEO, discusses crucial developments happening for Zeneo, a needle-free injector, functioning intramuscularly to administer medication in a tenth of a second.

This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.
Spain-based Inbrain Neuroelectronics plans first-in-human study to show safety of its graphene-based technology in direct contact with human brain while also developing a second interface for treating Parkinson’s disease.
The US Food and Drug Administration released three warning letters last month, two of which went to Chinese device testing labs accused of improper treatment of laboratory animals.
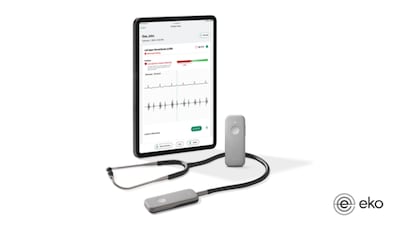
The results from a trio of studies show that AI-powered digital stethoscopes are effective at identifying patients at elevated risk of experiencing heart attacks and other major cardiac adverse events, according to Eko Health, whose technology was used to screen the participants in the studies.

Biopharmaceutical giant AstraZeneca has partnered with start-up “unicorn” Owkin to develop an AI-powered tool to prescreen for gBRCA mutations on the basis of morphological features in digitized pathology slides. Built on extensive, high-quality data sourced from the France-based PortrAIt consortium, the AI will help to prioritize patients for further testing, streamlining the diagnostic process, Owkin says.
Genetic Analysis CEO Ronny Hermansen and Christina Casén, senior VP of clinical and medical affairs, discuss the company’s polymerase chain reaction (PCR)-based approach to gut microbiota profiling versus DNA sequencing, competitive landscape, and opportunities for supporting pharma R&D and assessing drug treatment success.