ADVERTISEMENT
Behavioral Health

Medtech Insight was on the ground at HLTH looking for innovative companies. Learn about seven start-ups using AI to help physicians detect conditions including prostate and breast cancers, seizures and heart failure; assess patients for cognitive decline validate and deploy algorithms, and monitor patients in and out of the hospital.

Start-ups pitched a diverse deck of innovative technologies to three judges and an audience of potential investors, strategics and physicians at the Octane Medical Innovation Forum in Irvine, CA. Highlights include neuromodulation company Sinaptica Therapeutics, which won the competition for both “People’s Choice” and “Judge’s Choice.”

Altoida CEO Mark Jones has high hopes that the company’s digital assessment tool will be approved by the FDA to be used along with blood biomarker testing by primary care doctors to help predict Alzheimer’s disease before patients show symptoms.

Altoida CEO Marc Jones spoke with Medtech Insight about the company’s investigational digital screening tool for Alzheimer’s and the dire need for better, more accessible precision neurology diagnostics as the global population ages, neurologist shortages worsen, and groundbreaking Alzheimer’s drugs change the treatment paradigm.

This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.

Highlights from Medtech Insight's on-the-ground coverage of LSX in Boston.

The DBS trial will look at the use of neurostimulation in people with treatment-resistant depression, which affects about 2.8 million Americans each year. The implanted device may relieve depression symptoms by changing activity patterns in the brain.

In this week’s Digital Health Roundup, Medtech Insight’s Ryan Nelson highlights Click Therapeutics’ FDA-cleared digital therapeutics (DTx) for depression and Sinaptica Therapeutics’ personalized neuromodulation for Alzheimer’s patients. Marion Webb discusses her interview with MindMaze’s John Krakauer on their gaming-focused DTx to help people recover from serious brain injuries. Elizabeth Orr introduces new voting members of the new Digital Health Advisory Committee and Natasha Barrow discusses Hello Heart’s new symptom-tracking feature in their heart-focused app.
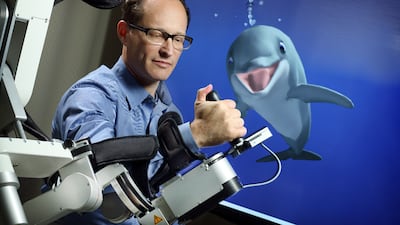
MindMaze seeks to create a beautiful immersive experience for patients recovering from brain injuries. Chief medical director John Krakauer, clearly a “Star Wars” fan, discusses the significant need for digital therapeutics at a time when therapists are in short supply, as well as the challenges facing the DTx space as a whole.

This week, Medtronic recalled a nerve monitoring system due to reports of false responses. The US FDA approved the first auto-injector for opioid-overdose, made by Purdue Pharma. The agency granted de novo authorization for Labcorp’s PGDx elio plasma focus Dx used by labs for genetic profiling. As of 7 August, 950 AI/ML devices have been approved by the FDA. EKO Health teamed up with LSU to help detect arrhythmias and murmurs in student-athletes.