Pathways & Standards
Approval Standards

Japan is cautiously easing Japanese clinical data requirements for rare disease drugs to allow faster and more flexible approvals, including on a conditional basis supported by postmarketing studies.

Woodcock and the Haystack Project want to modify a foundational concept of modern FDA drug efficacy assessments, which may be seen as an attempt to more formally codify and define regulatory flexibility.

Lyfgenia and Zynteglo comprise complex mixtures of transduced cells that represent different active ingredients, the company said in administrative appeals to the FDA. Two lawmakers also raised bipartisan concerns about the agency’s decision on the voucher request for the sickle cell disease treatment.

Remarks at the NORD Breakthrough Summit from the FDA’s Lola Fashoyin-Aje may suggest the agency is becoming more comfortable with single-arm trials.
Review Pathways

The Pharmacy Compounding Advisory Committee unanimously voted to add hydroxyprogesterone caproate products for reducing the risk of recurrent singleton spontaneous preterm birth to the Withdrawn or Removed List under sections 503A and 503B of the Food, Drug and Cosmetic Act.

Sponsors of generic drug applications that miss a goal date, but do not receive an action because of complex scientific or legal questions, would get a notice outlining the lingering issue as part of a new pilot program that might become permanent in the next review cycle.

Japan is cautiously easing Japanese clinical data requirements for rare disease drugs to allow faster and more flexible approvals, including on a conditional basis supported by postmarketing studies.
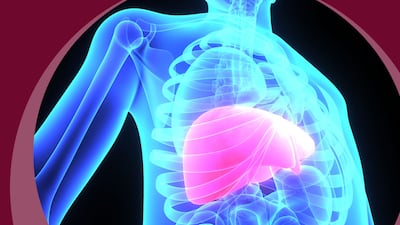
New nods for Boehringer Ingelheim’s survodutide, Sagimet’s denifanstat and Mirum’s volixibat bring the FDA’s non-infectious liver disease breakthrough therapy designations to 15, with six designees already approved.
User Fees

Another record-breaking year for novel approvals looks out of reach, but the six novel agents with November goal dates show the continued strength of rare disease drug development.

The number of standard and priority reviews also decreased significantly in FY 2023 compared to the previous year, which caused the fee for redeeming a voucher to rise.

Advisory committee concerns cast clouds over Iterum’s oral antibiotic, Intercept’s Ocaliva, and perioperative immuno-oncology regimens, while CSL and Pfizer aim to take their hematology franchises in new directions.

Inflation accounted for a larger portion of user fee revenue target increases for fiscal year 2025, compared to previous years, according to a Pink Sheet analysis.