Policy & Regulation

Sponsors of generic drug applications that miss a goal date, but do not receive an action because of complex scientific or legal questions, would get a notice outlining the lingering issue as part of a new pilot program that might become permanent in the next review cycle.

Respondents to the FDA’s questions over biosimilar development did not hold back. So, what is it: product-specific or product class-specific guidance? Or nothing?
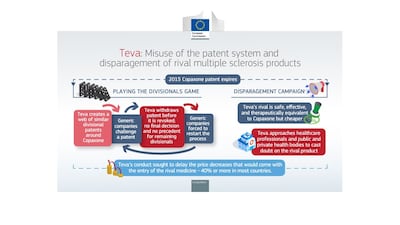
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

In a citizen petition to the FDA, Alvotech has called on the US agency to refrain from approving as interchangeable certain Stelara biosimilars that use a different cell line to its own ustekinumab product – including the Pyzchiva version set to be brought to market by Samsung Bioepis and Sandoz.
After sharing multiple updates to the regulatory status of its ketamine formulation, PharmaTher received a complete response letter from the FDA, which required minor information and clarifications from the company.

Only a month after filing their second biosimilar application with Japanese regulators, Alvotech and Fuji have submitted a further one for an undisclosed biosimilar as part of their local partnership.
French industry association Gemme has issued a stark warning that recent legislative proposals – including a revision to the country’s clawback mechanism and steeper financial penalties for failing to hold sufficient safety stocks – will “bring about the end of the generic economic model in France.”

With a looming deadline of 1 January 2025 for firms supplying Northern Ireland to comply with the Windsor Framework, UK generics and biosimilars association the BGMA has warned of potential supply interruptions due to requirements that include a “UK only” label for all packs as well as UK-based batch testing for biologicals.

Along with other approaches, early market entry of generics and biosimilars would barely make a dent in reducing the net prices of retail prescription drugs in the US – but even then, small reductions would add up to billions, reveals the latest CBO calculation.

With the UK generics market facing a rising number of supply issues, local off-patent industry association the BGMA has put forward a raft of policy proposals that it said could provide solutions.

The Philippines medicines regulator explains how it intends to implement the ASEAN mutual recognition agreement under which member states have committed to accept bioequivalence study reports for generics issued by approved BE centers.
The proposed CMS innovation center's $2 drug list model will not address the barriers to newer generics getting on Part D formularies and plans may have little incentive to participate in the demo, an industry group said.

Teva has resolved a pair of civil US Department of Justice lawsuits accusing the firm of violating the US Anti-Kickback Statute and the False Claims Act through its alleged conduct conspiring to fix the price of three generic drugs and for allegedly paying Medicare patients’ copays for its multiple sclerosis brand Copaxone.

The US FTC and FDA both received letters from the Senate but with different messaging. One commends for achieved findings and requests a new investigation and another scalds for not doing the assigned job.

Japan has started to charge patients a portion of the difference between the reimbursement price of the generic and non-generic product if they insist on the latter without a supporting recommendation from the prescribing physician, in a policy designed to further drive generic use.

A total of 748 key medicines are now affected by the four-month stock requirement, compared with 422 in 2021.

Generics Bulletin reviews several critical US bills and the off-patent industry’s response, along with budget calculations and new proposals.

Iconovo’s efforts in bringing its Breo Ellipta rival to the market continue, with positive news received from the FDA on the substitutability of the firm’s ICOpre proprietary inhaler. The firm is now seeking a partner to take the product to the next stage.

The US FDA has issued a Form 483 to Granules India, with six observations, spanning document and vent mismanagement to bird droppings and rust.

Generics Bulletin recaps the most recent regulatory news from across the world.