Regulation

Sponsors of generic drug applications that miss a goal date, but do not receive an action because of complex scientific or legal questions, would get a notice outlining the lingering issue as part of a new pilot program that might become permanent in the next review cycle.

Respondents to the FDA’s questions over biosimilar development did not hold back. So, what is it: product-specific or product class-specific guidance? Or nothing?
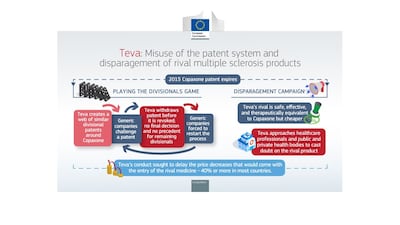
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

In a citizen petition to the FDA, Alvotech has called on the US agency to refrain from approving as interchangeable certain Stelara biosimilars that use a different cell line to its own ustekinumab product – including the Pyzchiva version set to be brought to market by Samsung Bioepis and Sandoz.
After sharing multiple updates to the regulatory status of its ketamine formulation, PharmaTher received a complete response letter from the FDA, which required minor information and clarifications from the company.

Only a month after filing their second biosimilar application with Japanese regulators, Alvotech and Fuji have submitted a further one for an undisclosed biosimilar as part of their local partnership.

Years after the patent application’s initial filing in 2007, the Indian Patent Office has dismissed ViiV’s claims in response to multiple opposition filings launched against the pharma player.

With a looming deadline of 1 January 2025 for firms supplying Northern Ireland to comply with the Windsor Framework, UK generics and biosimilars association the BGMA has warned of potential supply interruptions due to requirements that include a “UK only” label for all packs as well as UK-based batch testing for biologicals.

The Philippines medicines regulator explains how it intends to implement the ASEAN mutual recognition agreement under which member states have committed to accept bioequivalence study reports for generics issued by approved BE centers.

A total of 748 key medicines are now affected by the four-month stock requirement, compared with 422 in 2021.

Generics Bulletin reviews several critical US bills and the off-patent industry’s response, along with budget calculations and new proposals.

Iconovo’s efforts in bringing its Breo Ellipta rival to the market continue, with positive news received from the FDA on the substitutability of the firm’s ICOpre proprietary inhaler. The firm is now seeking a partner to take the product to the next stage.

The US FDA has issued a Form 483 to Granules India, with six observations, spanning document and vent mismanagement to bird droppings and rust.

Generics Bulletin recaps the most recent regulatory news from across the world.
At the recent Morgan Stanley Healthcare Conference in New York, Sandoz leaders set out their views on the significance of – and the FDA’s initial implementation of – the US biosimilar interchangeability designation.

Generics Bulletin’s editorial team discusses recent conferences held by Medicines for Europe and the AAM, while looking ahead to the key off-patent industry events that are on the calendar over the next few months and beyond.

Teva requested the change to the draft guidance, arguing that otherwise the meetings would be “completely one-sided conversations.”

The latest batch of product-specific guidances for generic development has been published by the US Food and Drug Administration, covering products that include COVID-19 treatment Paxlovid, ear drops and various metered-dose inhalers.

Generics Bulletin reviews the latest regulatory events across the world.

A better balance needs to be struck in allocating resources between Europe’s centralized and decentralized procedures for generic approvals, Medicines for Europe has urged, pointing to current limitations on member states acting as reference member states for decentralized procedures.