Regulation

Dietary supplement ingredient promising enhanced memory and mental clarity marketed widely across the US has been authorized for sale in Europe.

Trade group ready to work hand-in-hand with agency and other supplement industry stakeholders on potential regulatory changes or improvements, says president and CEO Steve Mister. “None of them are upsetting the basic balance of things that DSHEA was attempting to do, but there are things with 30 years that we've identified that need to be kind of fixed.”

New Jersey Assembly passed bill amended to expand extensively in defining which products, or ingredients, would be subject to a ban on sales to consumers younger than 18.

In first of two articles from recent interview, president and CEO Steve Mister discusses examples the trade group provides for self-regulation, “where people or companies who might not do it on their own because it would put them at a disadvantage if they were the only ones.”

EFSA has published new guidance to assist companies navigating the EU’s novel food authorisation process. HBW Insight speaks to experts Dr Mari Eskola of Medfiles and Dr Jérôme Le Bloch of FoodChain ID who offer differing views on the benefits and drawbacks of the guidance for industry.

Provision in Wyden and Merkley’s “Cannabinoid Safety and Regulation Act” referencing FDA authority outside hemp-derived ingredients could provide agency with needed authority to force from the market products labeled as supplements but containing drugs or eliminate the use of many safe ingredients in supplements.

Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”

Food Standards Agency tells companies marketing caffeine supplements, “It is your responsibility to ensure food supplements you sell are safe for human consumption.”

Brands also making market moves as lawmakers consider legislation instructing Transportation Security Administration to provide guidance to minimize risk for contamination of baby formula and related pediatric nutritional products.
Council for Responsible Nutrition contends New Jersey bill, which Assembly Health Committee amended with substantial language to make restrictions more stringent on 23 September, is targeted as inaccurately as restrictions in New York effective in April.
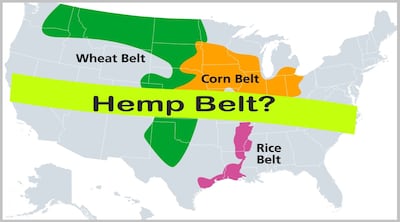
Democrats Wyden and Merkley author Cannabinoid Safety and Regulation Act to limit sales to consumers 21 and older and authorize FDA to order recalls and impose bans on cannabis products with dangerous chemicals or additives. It also would establish regulatory structure for using cannabinoids found naturally in hemp and allowing “semi-synthetic” ingredients while prohibiting artificial or fully synthetic cannabinoids.

Following Energy and Commerce Health Subcommittee hearing about FDA’s human food and tobacco programs on 10 September, gap between what the trade groups, committee leadership and the FDA each want more of doesn’t appear to be shrinking.

The Federal Trade Commission’s Consumer Protection head Sam Levine says bipartisan leaders at the state and federal levels are taking on consumer protection issues like never before and using the FTC’s approach as a model for legislation.

In addition to commentary and information about the OTC drug, dietary supplement and cosmetic industries, speakers offered candid remarks on subjects well known across the consumer health industry or well recognized by consumers.

Escaping A Regulatory Labyrinth: France Adopts Streamlined Approach To Food Supplement Notifications
Companies launching food supplements in France must now navigate a new product notification platform. EcoMundo’s Corinne Rayapin discusses why the change is happening and the expected benefits for both industry and consumers.

Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.

In this installment of HBW Insight’s “Inside Regulatory Affairs” series, Haleon’s regulatory strategist Niels Kildemark welcomes the challenges that the EU Green Deal brings, but warns that meaningful consultation with all stakeholders, including industry, must be prioritized to ensure clarity of regulatory purpose and execution.

Reorganization creating in Human Foods Program makes the work of additional staff available for dietary supplement office programs. FDA’s “got a little tiny workforce” for “a huge industry,” says Commissioner Robert Califf.

Germany's BfR says certain groups, including pregnant women and those with certain health conditions, should not use ashwagandha supplements, while the rest of the population should exercise caution due to the potential risks.

“It's really the elected officials who are making the final determination on what happens. They're really the owners of the teams, you guys in the industry are playing the game we’re refereeing,” FDA Commissioner Robert Califf says at Consumer Healthcare Products Association conference.