Asia

The move towards greater regulatory collaboration is a positive step for gene therapy developers, Astellas’ gene therapy strategy lead Richard Wilson says – adding, however, that pharma still needed to understand the Asian markets better.

Japan is cautiously easing Japanese clinical data requirements for rare disease drugs to allow faster and more flexible approvals, including on a conditional basis supported by postmarketing studies.

Dispute around the first-in-world license for a self-amplifying mRNA COVID-19 vaccine castes light on Japan’s ongoing dilemma between a government trying to build its own capabilities to attract, develop and manufacture new modalities and the anti-vax movement in the country.

Whether the legislation aimed at discouraging use of Chinese contractors passes in its current form or not, industry will continue to face more pressure to decouple its operations.
Europe
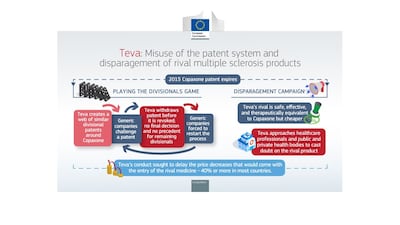
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

Companies that delay their drug application submissions to the European Medicines Agency by more than 60 days will face an additional fees of €4,200 per delay under a new regulation effective from 2025.

The European Medicines Agency is conducting a public consultation on proposed revisions to its policy on how it handles any conflicts of interest of its scientific committee members and experts.

The move towards greater regulatory collaboration is a positive step for gene therapy developers, Astellas’ gene therapy strategy lead Richard Wilson says – adding, however, that pharma still needed to understand the Asian markets better.
International

The move towards greater regulatory collaboration is a positive step for gene therapy developers, Astellas’ gene therapy strategy lead Richard Wilson says – adding, however, that pharma still needed to understand the Asian markets better.

The Pink Sheet highlights recent comments and insights from pharma officials and executives on key issues we are covering.

Meeting the regulatory gold standard for drug candidates in ultra-rare diseases can be impractical, a regulatory expert says, but greater collaboration and shared insights from regulatory reviews could help find a viable path forward.

Decentralized manufacturing methods for cell and gene therapies will be critical for improving patient access to treatments, but sponsors must prepare to demonstrate “comparability” with centralized manufacturing.
Latin America
The deal comes at a time when Latin American countries are increasingly looking to improve the regulatory environment for pharmaceuticals and move towards greater convergence.
Mexico is to establish a new regulatory framework that is in line with international standards to encourage domestic production of biosimilar medicines.

El Salvador has approved a law that will establish a new regulatory body responsible for authorizing medicines and setting prices.

Mexico aims to speed up the registration of generics and biosimilars.
Middle East & Africa

A newly published draft implementing act sets out the procedural rules for the joint scientific consultations that are foreseen by the EU’s Health Technology Assessment Regulation.

The World Health Organization has also prequalified the Bavarian Nordic vaccine to enable broader and timely access.

Companies marketing pharmaceutical products in Saudi Arabia are being urged to prepare for upcoming mandatory pharmacoeconomic assessments to demonstrate the added value of their drugs over existing treatments.
The Democratic Republic of Congo, the country worst hit by the mpox crisis, has now received the first batch of vaccines for the disease. Meanwhile, the World Health Organization is expected to complete its review for emergency use listing of mpox vaccines soon.
North America

Former Food and Drug Law Institute CEO Amy Comstock Rick will take on patient engagement for the US FDA Rare Disease Hub as director of strategic coalitions.

US FDA’s Rare Disease Innovation Hub should shepherd the practical transformation of the diverse wealth of patient-generated data held by advocacy organizations into information for regulatory use, Reagan-Udall public meeting hears.

Republican presidential candidate Donald Trump says he will let Robert F. Kennedy Jr. “go wild on medicines” if he wins the White House. That could spell challenges for the US FDA in 2025.

Lexicon’s Zynquista: Negative Adcomm Vote In Type 1 Diabetes Includes Support For Revised Indication
Some panelists favored use in T1D patients with mild chronic kidney disease, even though that was not the original indication Lexicon proposed, which was rejected in an 11-3 vote.