
The move towards greater regulatory collaboration is a positive step for gene therapy developers, Astellas’ gene therapy strategy lead Richard Wilson says – adding, however, that pharma still needed to understand the Asian markets better.

Japan is cautiously easing Japanese clinical data requirements for rare disease drugs to allow faster and more flexible approvals, including on a conditional basis supported by postmarketing studies.

Dispute around the first-in-world license for a self-amplifying mRNA COVID-19 vaccine castes light on Japan’s ongoing dilemma between a government trying to build its own capabilities to attract, develop and manufacture new modalities and the anti-vax movement in the country.

Whether the legislation aimed at discouraging use of Chinese contractors passes in its current form or not, industry will continue to face more pressure to decouple its operations.

A court order encompassing funding, drug pricing, clinical trials and overall policy implementation aspects is expected to cause a paradigm shift in the treatment of rare diseases in India. Sarepta, Roche and Sanofi are among the companies that have been part of pricing discussions.

China recently made strategic moves to allow direct foreign investments into cell and gene therapy development, as well as into hospitals in certain cities and regions across the country. Foreign firms are generally optimistic despite some challenges.

With the US BIOSECURE Act waiting for a Senate vote, there are signs it may be prompting some Chinese firms to look at their operations. In the meantime, two legal experts in China suggest a range of coping strategies for companies that may be deemed "of concern."

Contract research organizations in India must gear up to comply with new registration requirements coming into effect in April 2025 that aim to enhance the quality and integrity of clinical trials, as well as of any bioavailability and bioequivalence studies conducted by them.

Japan has started to charge patients a portion of the difference between the reimbursement price of the generic and non-generic product if they insist on the latter without a supporting recommendation from the prescribing physician, in a policy designed to further drive generic use.
The US FDA Commissioner pushed for rebalancing the US’s pharmaceutical supply chains while also stressing that US-China commerce has a role that would be risky to compromise.

Japan plans to offer wider support to foreign firms and ventures with innovative candidates to start clinical trials in the country, as part of key measures from current prime minister Fumio Kishida.

With BIOSECURE's legislative progress on pause until after the election, a Pink Sheet infographic looks back on the Capitol Hill progress to date and looks ahead to the potential impact if it is enacted, using Evaluate Pharma data to highlight the likely holes in pharma’s supply chain.

The five-year roadmap aims to expand support for AI research and development in essential health care and new drug development, as well as advance medical data usage systems and enable its safe use.
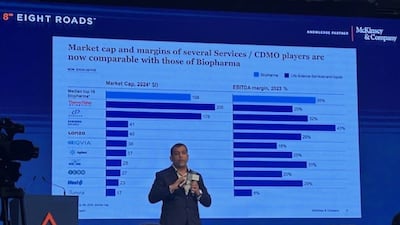
A senior McKinsey executive discusses trends in the CDMO sector amid geopolitical tensions, the spurt in customer queries at Indian firms and also facets of the deals scenario.

With the BIOSECURE Act halfway to congressional approval, stakeholders are pushing for a financial boost to ensure the US gains the business Chinese companies will lose, but that may be a big lift.

Non-Japanese firms without offices in the country may submit documents for approval filings in English, subject to certain conditions.

The Pink Sheet Drug Review Profile explores the US FDA’s approval of vadadustat to treat anemia in chronic kidney disease patients on dialysis. A complete response letter cited the risk of drug-induced liver injury, but postmarketing data from Japan reassured reviewers.

Developments in India and Pakistan are designed to standardize how drug companies disclose their expenses associated with health care professionals.

Under the new drug approval innovation measures, Korea will cut the review and approval period of new drugs to 295 days from 420 days and increase the number of expert reviewers to enhance review capabilities.
If the US Congress continues to target trials at Chinese military hospitals, the impact could be relatively small, while a broader focus could upend multinational development programs.